![]() What is Spray Drying?
What is Spray Drying?
Spray drying has become an invaluable technique in the pharmaceutical world, particularly for transforming liquid formulations into dry powder, a critical step in developing inhalable therapeutics. Fast and cost-effective, spray drying allows precise control over particle characteristics such as size, stability, and solubility, making it ideal for producing high-quality, consistent powders for dry powder inhalation (DPI) products.
At Experic, our spray drying expertise shines in controlling particle size and other attributes to ensure that each particle behaves as intended within the respiratory system. By using advanced techniques to fine-tune particle size, we enable precise lung targeting, manage cohesiveness to prevent aggregation and ensure consistent dosing. Our team also excels in encapsulation and blending, combining these capabilities for optimal outcomes
How Does Spray Drying Work?
Spray drying is used to optimize particle characteristics for consistent and effective medication delivery and to improve formulation stability, ensuring reliability and efficacy over time. But how exactly does spray drying work?
Spray drying pumps a liquid formulation through a spray nozzle or atomizer, where it is converted into small droplets. The atomized droplets are then introduced into a drying chamber or tower. Here, they come into contact with a stream of hot air or gas for rapid evaporation of the liquid component, leaving behind the powder formulation. The particle size can be fine-tuned through the atomization process parameters and solvent, allowing for the production of powders with specific particle size distributions. This attribute can influence the respirability, dissolution rate, bioavailability, and other characteristics, improving its suitability for a DPI product.
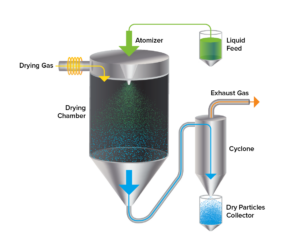
Spray drying can also be used to improve the stability of certain pharmaceutical compounds by removing water or other volatile solvents as compared to a solution-based formulation. Water removal can reduce the requirement for aseptic processing in nonsterile applications due to the reduction in free water and thus, water activity, while the resulting dry powder can have a longer shelf life and increased resistance to degradation.
Finally, spray drying can be employed to improve the solubility and dissolution properties of poorly soluble drugs. By formulating the drug in an amorphous solid dispersion with certain excipients, the drug’s bioavailability can be enhanced.
Ensuring Quality
Quality by Design (QbD) is a proactive approach to building quality into product development, enhancing capability, speed, and formulation design. It is a technique that increases efficiency by focusing on the most important interactions while appropriately managing potential risk.
Spray drying optimization involves assessing various interrelated factors. For instance, when optimizing atomization, factors like liquid feed properties such as viscosity and temperature, as well as the design and configuration of the atomizer, must be considered to achieve the desired droplet characteristics. To reduce the number of experiments needed to achieve critical quality attributes (CQAs), pilot-scale spray drying programs can be employed.
Optimization requires evaluating multiple factors that interact with each other and can have an impact on the CQAs of the dry powder, including particle size distribution, density, morphology, and flowability; factors that are important when formulating powders for inhaled delivery.
Use of a QbD approach is essential during process development activities whereby statistical methods can increase process understanding and the impact of process controls. Partial-factorial and definitive study experimentation can also help to reveal interactions and reduce the number of experiments to achieve desired CQAs.
Advancing DPI Development
Particle size and cohesiveness can impact DPI development, directly influencing bioavailability and delivery efficiency. Furthermore, biologic molecules present further challenges to DPI development, requiring a deep understanding of both the physical properties of the drug and its regulatory requirements for successful formulation. Partnering with an expert Contract Development Manufacturing Organization (CDMO) can give DPI developers a leg up in formulation and development, streamlining the path to market for innovative new therapies.