EU Clinical Supply Center
Strategically Located for Global Access
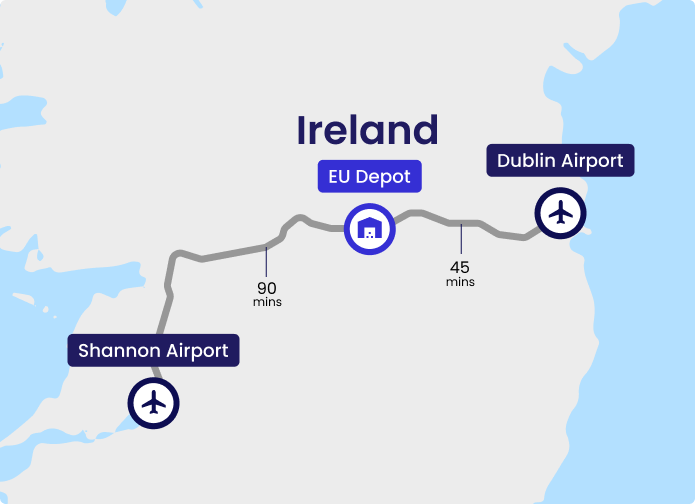
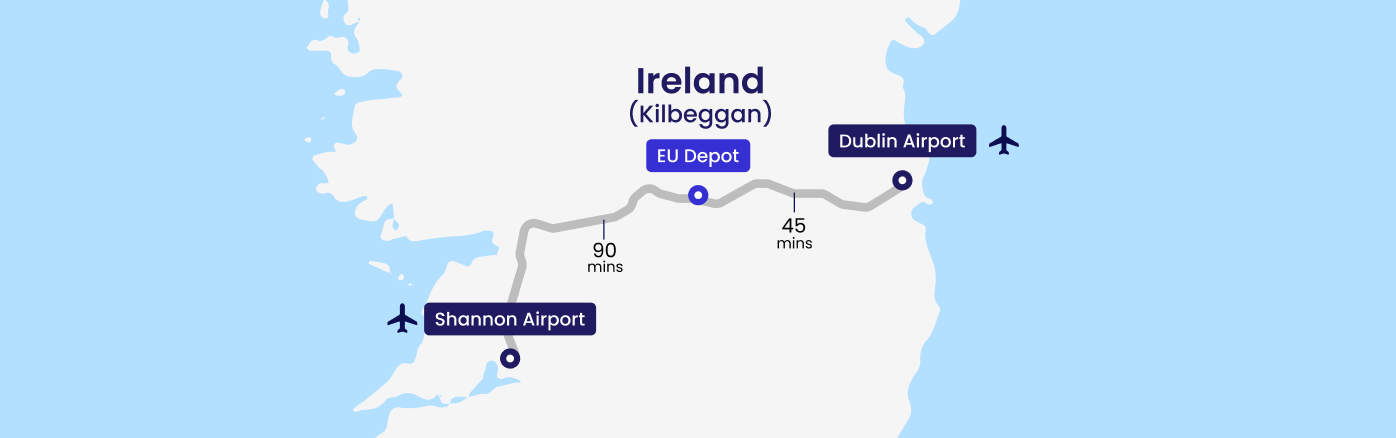
Experic’s EU Clinical Supply Center facility in Kilbeggan, Ireland, offers world-class clinical supply, packaging, and distribution services. Centrally positioned in Ireland, it is just 45 minutes from Dublin Airport and 90 minutes from Shannon Airport, providing easy access for global pharmaceutical logistics.
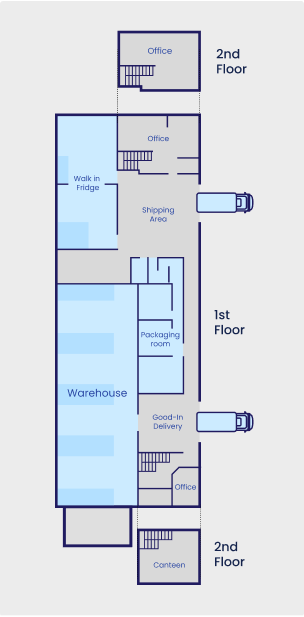
Our 700 m² facility is designed to support your clinical trials. It offers specialized storage, packaging, labeling, procurement, returns, QP, and distribution services to ensure product integrity and regulatory compliance.

Storage & Distribution Services

Experic’s EU Clinical Supply Center offers a full range of temperature-controlled storage solutions to ensure the integrity of pharmaceutical products throughout the clinical supply chain:
- +15°C to +25°C (Ambient)
- +2°C to +8°C (Refrigerated)
- -25°C to -15°C (Frozen)
With advanced monitoring and security, we ensure that your products remain in optimal condition throughout storage and distribution.
Specialized Packaging & Labeling Services

Experic’s EU Clinical Supply Center offers a full range of temperature-controlled storage solutions to ensure the integrity of pharmaceutical products throughout the clinical supply chain:
- Three dedicated rooms for secondary packaging and labeling at +15°C to +25°C
- Two controlled rooms for secondary packaging and labeling at +2°C to +8°C
We provide full-service clinical trial packaging solutions, including customized labeling, kitting, and serialization to support global supply chain needs.
Qualified Person (QP) Services
Experic’s EU Clinical Supply Center provides expert QP services to ensure the highest standards of compliance for clinical and commercial products:
-
Ensuring compliance with Good Manufacturing Practices (GMP).
-
Facilitating seamless international drug product movement.
-
Expediting product release for clinical and commercial use.
With our experienced QP team, we streamline regulatory approvals and ensure compliance with EU Good Distribution Practice (GDP) and Good Manufacturing Practice (GMP) guidelines.
Procurement & Clinical Trial Returns Management
Experic’s EU Clinical Supply Center supports end-to-end clinical trial supply needs with specialized services in:
-
Procuring clinical trial materials from approved global suppliers.
-
Managing trial materials with strict compliance and efficiency.
Our procurement and returns management services help sponsors reduce risk, optimize trial logistics, and maintain regulatory compliance throughout the clinical supply chain.
Specialist Services
Experic offers bespoke development, manufacturing, and clinical packaging, labeling, storage, and distribution services to meet your product's unique needs.