The pharmaceutical industry is on an upward trajectory, projected to reach US$1,454.00bn by 2029. Artificial intelligence (AI) is playing a pivotal role in this growth, offering tools to streamline operations, monitor regulatory changes, and even predict potential issues like equipment failures and product defects. Research also suggests that AI is a powerful tool for optimizing company spending. Market predictions estimate that in five to seven years, cost savings are expected to increase to a range of 22%-33% with cost savings increasing to 44%-67% once peak adoption of AI is reached. But is AI just a trend, or does it have a lasting place in our industry? In this blog we will explore the true impact of AI in quality control and regulatory compliance.
AI’s Role in Quality Control and Operations
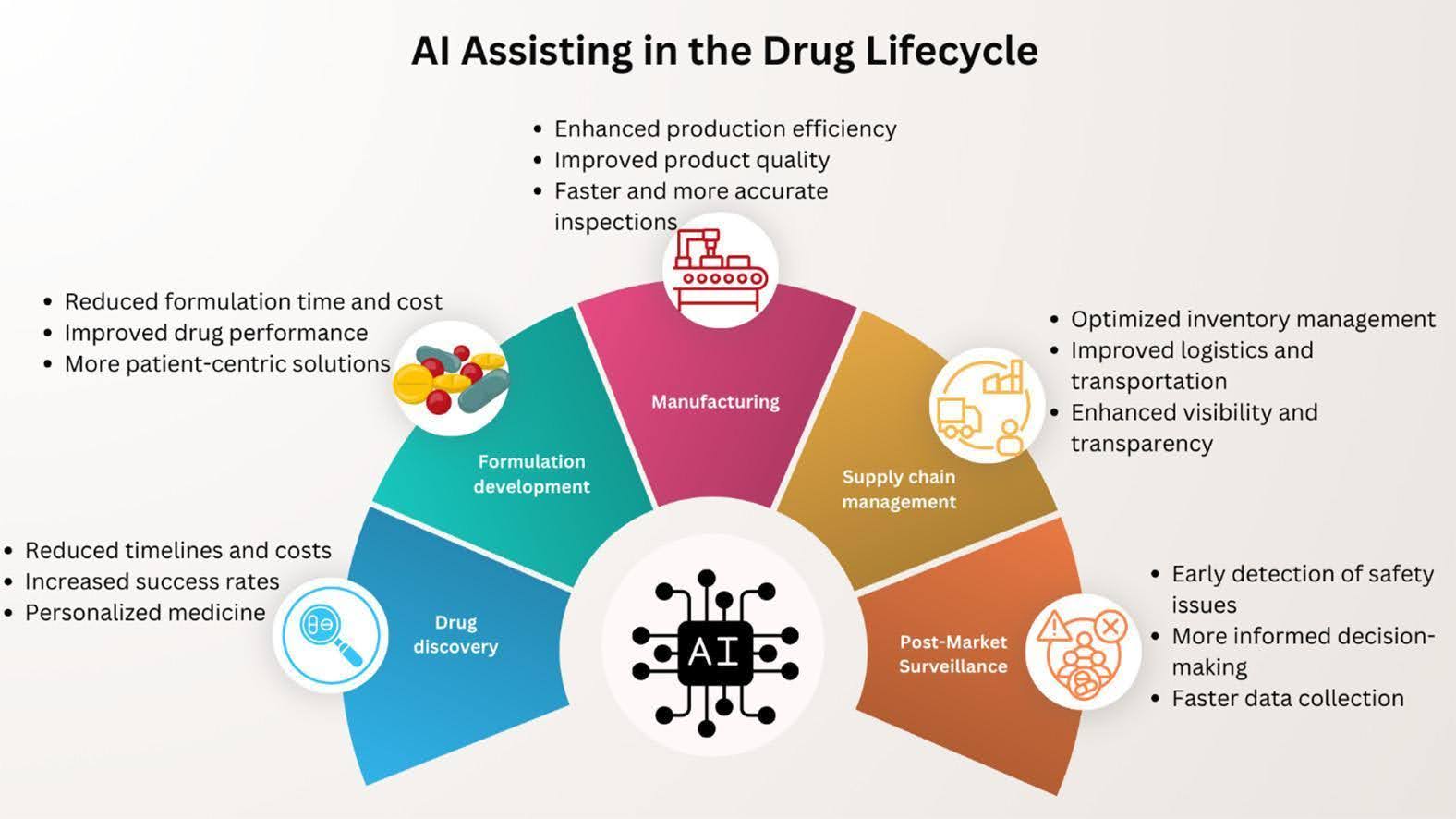
Source: Huanbutta et al. (2024) European Journal of Pharmaceutical Science
Recent studies highlight how AI is pharmaceutical manufacturing services. A review in the European Journal of Pharmaceutical Science reveals how AI enhances real-time inspection, reduces errors, and optimizes manufacturing processes. Key benefits include:
- Improved decision-making through advanced data analytics.
- Enhanced monitoring of products, ensuring consistent quality.
- Streamlined tasks, reduced waste, and better production outcomes.
- Simplified adherence to complex regulatory standards.
- Ongoing process optimization for sustained improvements.
AI has been successfully used in regulatory compliance, PharmaPhorum notes two examples of leading pharmaceutical companies that used AI to enhance their processes. The first, a biopharma product development and supply group, built a data lake to integrate internal (e.g., CAPAs, risks, RTQs) and external (e.g., FDA letters, BLAs) data sources in order to improve risk management across formulations, supply, and regulatory compliance. Using Natural Language Processing (NLP), the company structured and extracted insights on concepts, relationships, and sentiments within the data. Visualizations provided end-users with actionable intelligence, and automated workflows ensured up-to-date, scalable reporting on regulatory risks and recommendations.
Another pharmaceutical company addressed this inefficiency by implementing an NLP-powered workflow integrated with Large Language Models (LLMs). This approach created a regulatory intelligence assistant that offered Q&A-style access to up-to-date regulatory insights. The tool also categorized risks and summarized data on user-specified substances, enabling dynamic, efficient monitoring of regulatory landscapes and enhancing decision-making capabilities.
Navigating AI Regulations
The adoption of AI in pharmaceutical development services isn’t without challenges, particularly regarding compliance with rapidly evolving regulations. The European Union’s AI Act, effective from August 2024 requires manufacturers to meet the acts requirements by 2025 or face fines up to €35 million for noncompliance. Meanwhile, the U.S. FDA is introducing AI initiatives that explore in silico applications while also seeking to address potential biases. Luckily, AI itself is increasingly helping companies to stay up to date and even get ahead of these evolving regulations. Here are two ways that AI is being used to enhance regulatory compliance:
- Data-driven risk management: GSK implemented an automated compliance system that significantly reduced documentation errors during audits. This demonstrates how AI can enhance data accuracy and minimize compliance risks.
- Semi-automated regulatory intelligence monitoring: 90% of the world’s leading pharmaceutical and medtech companies already use AI to analyze trends from millions of data points gathered from regulatory and inspection agencies. It is estimated that this automation is saving hundreds of staff hours monthly in compliance risk reduction.
With the success of AI systems in these areas, many researchers hope that AI will soon address broader areas of regulatory labeling, intelligence and mapping.
Staying Ahead with AI
As drug development continues to focus on complex and less soluble drug development, staying up to date with global regulatory standards becomes increasingly challenging. AI helps bridge this gap by flagging nuanced regulatory changes, automating document translation, and accelerating tasks that once required weeks, completing them in days or even hours. While human expertise remains indispensable for verification and strategic decisions, AI empowers specialists to focus on higher-value tasks, while maintaining high-quality and regulatory compliance.
The Future of AI in Pharmaceutical Development
It’s clear that the integration of AI into the pharmaceutical sector is more than a passing trend. By enhancing quality control, pharmaceutical process optimization, and simplifying compliance with complex regulations, AI serves as a powerful tool that can help industry experts to be more efficient and productive. The benefits of AI in the pharmaceutical sector have yet to be fully seen but their advantages in quality control and regulatory compliance are crystal clear. Join us in embracing the benefits of advanced technologies to accelerate timelines and enhance patient outcomes.
Ready to experience the Experic difference? Contact us today to learn more about our AI-driven pharmaceutical contract manufacturing solutions and how we can help you achieve your drug product development goals.
References
- “Pharmaceuticals Worldwide.” Statista, 2024, https://www.statista.com/outlook/hmo/pharmaceuticals/worldwide.
- “How Pharma Can Benefit from Using GenAI in Drug Discovery.” EY Insights, 2024, (https://www.ey.com/en_us/insights/life-sciences/how-pharma-can-benefit-from-using-genai-in-drug-discovery#:~:text=Target%20identification%20(67%25%20reduction%20at,for%20resource%2Dintensive%20clinical%20trials).
- Huanbutta, Kampanart, et al. “The Artificial Intelligence-Driven Pharmaceutical Industry: A Paradigm Shift in Drug Discovery, Formulation Development, Manufacturing, Quality Control, and Post-Market Surveillance.” European Journal of Pharmaceutical Sciences, vol. 106, 2024, p. 106938.
- “Gaining a Better Understanding of Diseases We Want to Treat.” AstraZeneca, n.d., https://www.astrazeneca.com/r-d/data-science-and-ai.html#:~:text=Today%20we%20use%20AI%20to,therapeutics%20and%20cell%2Dbased%20therapeutics.
- “Implementation Timeline | EU Artificial Intelligence Act.” Artificial Intelligence Act, n.d., https://artificialintelligenceact.eu/implementation-timeline/.
- “Addressing the Limitations of Medical Data in AI.” FDA, https://www.fda.gov/medical-devices/medical-device-regulatory-science-research-programs-conducted-osel/addressing-limitations-medical-data-ai?utm_medium=email&utm_source=govdelivery.
- “How Pharma Can Improve Regulatory Compliance with AI-Based Technology.” PharmaPhorum, https://pharmaphorum.com/digital/how-pharma-can-improve-regulatory-compliance-ai-based-technology.