For any clinical trial, a reliable supply chain for investigational products and materials is essential to ensure that trials run smoothly, meet regulatory standards, and maintain the integrity of the data. To successfully achieve this, many clinical trial sponsors decide to partner with a specialized clinical trial supply service provider. Selecting the right partner for clinical trial supply (CTS) services, however, is a significant decision that can impact the timeline, costs, and overall success of your trial. Choosing the wrong CTS partner can introduce bottlenecks, product quality issues, and logistical nightmares that derail the study – so it’s crucial to understand the key considerations before entrusting someone with this critical function for our product’s success.
Why CTS Services Matter
CTS providers handle complex supply chain tasks, including the manufacturing, packaging, labeling, distribution, and safe return or destruction of trial materials. Their role is vital to maintaining regulatory compliance and adapting quickly to changes that may arise in trial timelines or patient enrollment. For these reasons, many sponsors partner with specialized CTS providers with the infrastructure and expertise to manage these complexities swiftly and effectively.
Key Criteria for Selecting a CTS Partner
- Proven Experience: Look for a CTS provider with a solid track record in managing clinical trial supplies across various phases and geographies. References from past clients and industry feedback are essential to gauge reliability and satisfaction.
- Comprehensive Services: A well-rounded CTS partner should offer services from end-to-end, including procurement, packaging, labeling, storage, and distribution. They should also excel in managing unforeseen circumstances, such as fluctuations in patient enrollment or changes in clinical sites. Contingency planning, secure storage, and effective returns management are key to ensuring uninterrupted supply.
- Regulatory Compliance: Clinical trials are subject to rigorous standards set by regulatory bodies such as the FDA and EMA. Your CTS partner should be proficient in Good Manufacturing Practices (GMP) and other international standards. This includes expertise in labeling controlled substances and highly potent drugs, as well as providing child-resistant packaging where required.
- Scalability and Flexibility: The right partner should have the capacity to scale their operations to accommodate the size and scope of your trial, regardless of its geographical reach. Flexibility to adapt to evolving needs is critical, as trial parameters can change rapidly.
- Quality Assurance: High standards of quality control are non-negotiable. A robust Quality Management System (QMS) ensures the consistency, safety, and reliability of trial supplies, and helps maintain compliance throughout the supply chain.
- Global Reach: For trials spanning multiple regions, a CTS provider with global logistics capabilities is invaluable. They should have a strategic network of distribution facilities and an understanding of regional regulations to avoid delays.
Getting ready to sit down with a CTS provider?
Download our comprehensive checklist to make sure you are fully prepared.
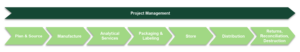
The CTS Workflow: From Planning to Completion
An effective CTS workflow is a multi-stage process involving thorough planning, precise sourcing, and timely manufacturing. A typical CTS process would look something like this:
- Planning & Sourcing: Establish the production schedule and coordinate inventory to ensure supply meets patient enrollment needs. This phase also focuses on implementing efficient inventory control and proactive risk assessment of supply scenarios and contingency planning.
- Manufacturing & Quality Control: Ensure chemistry, manufacturing, and controls (CMC) process development and compliance. Quality and analytical support includes critical items such as method development and validation, product release, and ICH-compliant stability testing.
- Packaging & Labeling: Customized packaging solutions are developed to meet the specific requirements of each clinical trial and ensures patient safety, timely and secure delivery to trial sites, and adherence to regulatory standards. A range of labeling options and translation services are also needed to address the needs of clinical trial locations in different countries.
- Distribution & Storage: Global logistics and temperature-controlled storage help maintain product integrity, from receipt and manufacturing to storage and distribution. In addition to maintaining product integrity through prequalified cold chain logistics, timely and reliable distribution to clinical trial sites or directly to patients is also a crucial component.
- Returns & Reconciliation: The retrieval, accounting, and disposal of drug products are essential final steps that guarantee compliance with regulatory standards and preserve the integrity of the clinical trial.
Overcoming Challenges in CTS
Budgeting, lead times, and complex logistics are common challenges in clinical trial supply management. A trusted CTS partner provides specialized expertise, global capabilities, and the flexibility to respond to changing clinical needs. Outsourcing these services allows sponsors to focus on their core competencies while ensuring efficient trial supply logistics, ultimately advancing new therapies to market more swiftly and safely.
At Experic, we’re committed to managing your CTS needs with precision and care. Our team offers comprehensive solutions for every stage of the clinical trial process, putting patients and trial integrity at the forefront. Learn more about our CTS services here.
Looking for more advice on choosing a partner or setting up a strong CTS program?
Read our White Paper